RINSW is capable to perform the whole breadth of imaging studies from high volume basic screening studies to complex and technically challenging basic research imaging. The unique location within the Prince of Wales Hospital allows for the recruitment of almost any participant with any needs. Full medical equipment including medical gases is available.
Instrumentation
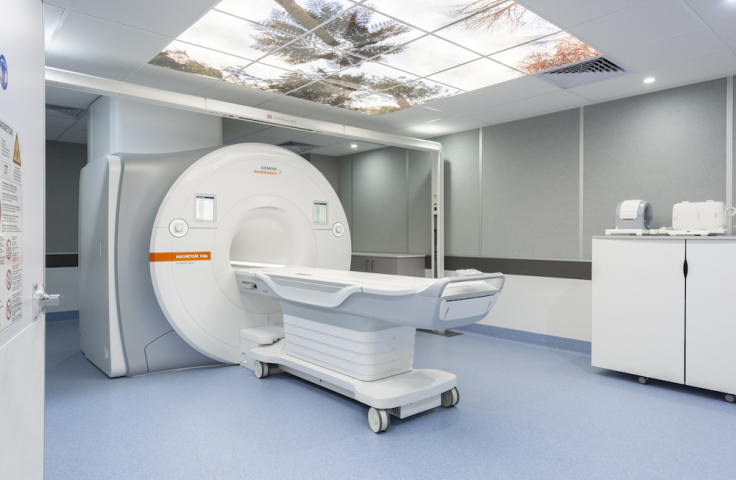
The Vida has a wide bore (70 cm) magnet design that is friendly for children and suited for anxious or larger participants. The room is equipped with medical gases and has a laser bridge for co-registration of radiation treatment. Acquisition and postprocessing licenses are available for most advanced techniques as well as a developer license to run customer-built sequences. In collaboration with Siemens, several works-in-progress packages (WIP’s) are installed in support of protocols utilizing advanced or emerging methods.
Technical details
The Vida is a 3T zero boil-off magnet with a 60 mT and 200 T/m/s slew rate gradient system. The RF system is optimized for 3T with multiple RF transmitters allowing field shaping. The system is equipped with 64 receive channels, a 64 channel head coil, 60 channel body coil and high density MSK coils and other specialty coils for a broad range of applications. It features the BioMatrix Technology, which enables physiological monitoring without bellows, ECG or pulse monitoring cables.
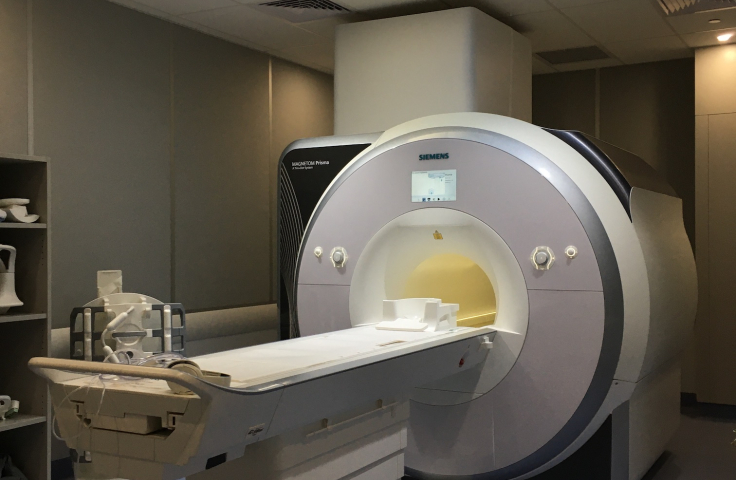
The Prisma has a standard bore size (60cm) but features higher gradient strength that is advantageous in advanced methods like DTI. A set of similar software licenses as for the Vida is available, including the developer license and WIP’s.
Technical details
The Prisma is a 3T zero boil-off magnet with 80mT/m gradients and a maximum slew rate of 200 T/m/s. The RF system is optimized for 3T with multiple RF transmitters allowing field shaping. The system is equipped with 64 receive channels, a 64 channel head/ neck coil, 32 channel spine and 18 channel body coils, as well as specialty coils for a broad range of applications.
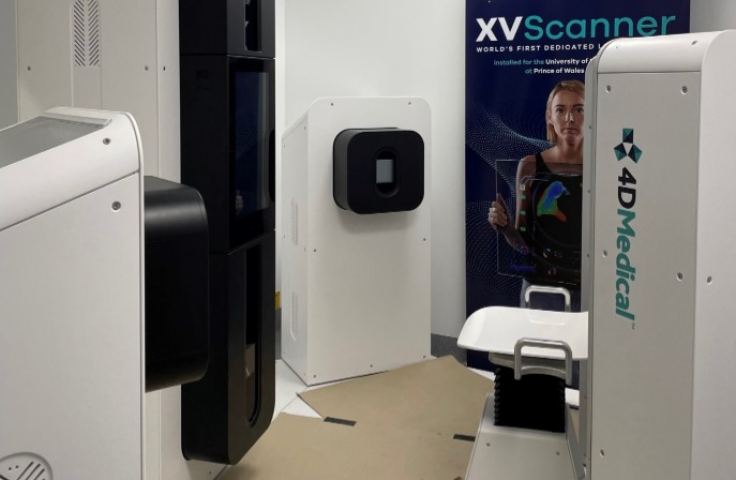
The XV scanner is the first human installation of a new X-ray based technology that aims to spatially visualize lung ventilation by acquiring time resolved x-ray projections simultaneously under different angles using multiple sources and detectors.
Research with children has found that using an MRI simulator before the actual scan provides a chance for children to experience an almost realistic MRI environment and complete the subsequent exam successfully. The MR simulator allows children to ask questions about their upcoming scan and to practice holding still. For children (as well as adults) who are afraid of going into an MRI scanner, MR sensitisation therapy may help to avoid having the exam under general anaesthesia. MR simulators are also very helpful to practice activation tasks in the context of functional MRI of the brain.
Our MRI simulator (PST-101728, Psycholgy Software Tools, USA) will be installed at the Sydney Children’s Hospital. The Team from Sydney Children’s Child Life and Music Therapy will lead the sensitisation therapy and assist with training sessions.
A multitude of auxiliary equipment is available in support of research studies. These include but are not limited to:
- Separate viewing station with advanced postprocessing licenses for image analysis
- Standard clinical contrast injectors (on Prisma and Vida)
- MR-conditional clinical life sign monitor for use with aneaesthesia shared between systems
- MR-conditional Pulse oximeter and pulse rate monitor (at Vida)
- MRE actuator (on Prisma and Vida) with a range of drivers (rigid driver and soft, i.e. flexible drivers in two sizes)
- Bold-screen setup with response boxes (on Prisma and Vida) for presentation of fMRI paradigms or entertainment of participant
- Presentation license
- Several other commonly used presentation packets are available and integrated (e.g., PsychoPy, PsychToolbox)
- An Eye tracker is available at the Prisma
- MR compatible goggles with a range of exchangeable prescription glasses for optimal visual stimulation of near- or far-sighted subjects
- A 64 channel MR-compatible EEG system with caps in different sizes and configurations
- An additional 128 channel MR-Compatible EEG system is shared with Neura and can be accessed upon request
- A 3D Printer with biocompatible materials is available to create project specific phantoms or other items
- Computer workstations can be used for e.g. postprocessing or sequence development (Siemens sequence development environment is available)
Research Imaging NSW uses Flywheel for processing and sharing data (DICOM and other). Flywheel allows for processing, visualization and quick & easy download of de-identified MRI research data. Each research group has access only to their own data and data access can be further restricted based on individual projects. Data can automatically be post-processed with flywheel and converted into other formats (e.g., NifTI, BIDS)*. Command line and API access is possible for batch-processing or integrating with other systems.
Data will generally be available for download within 24 hours after the scan. Access the RINSW instance of flywheel.
SESLHD and SCH patient data will be archived in the clinical PACS system and can be accessed from there by authorized health care providers.
*Additional or computational intensive processing may incur separate charges. Contact RINSW if advanced processing is needed.
RINSW has capabilities to assist researchers in sequence design, testing and implementation onto the facility's systems. An expert team of technical and scientific support staff comprised of Radiologists, Radiographers, an MR Physicist and a Siemens Clinical Scientist are available for advice and training.
Researchers are required to have the appropriate research ethics approval in place before scanning can proceed. The primary Human Research Ethics Committee (HREC) for this facility is the South Eastern Sydney Local Health District (SESLHD) HREC. UNSW researchers with research projects involving only healthy volunteers may be able to obtain approval from the UNSW HREC.
Please contact us if you require information on how to apply for ethics approval for research projects conducted at the facility.
All scans are read by a radiologist as part of the 'Duty of Care' process and will receive a 'Duty of Care' report. More information on the Duty of Care process at RINSW that ensures incidental pathological findings are addressed appropriately is available here.